Table of Contents
Introduction of Nuclear Energy
Nuclear energy is a form of energy stored within the nucleus of an atom and released during nuclear reactions like fission or fusion.
- It comes from the nucleus and the core of atoms, which are made up of protons and neutrons.
- Nuclear energy can be produced in two ways, that is, fission or fusion.
- It’s a carbon- free energy source that can be used to generate electricity.
- It is also the world’s largest source of emission- free energy.
- The most common nuclear fuels are 235U and 239Pu.
- The process of generating electricty by enriched uranium to create heat and heat creates steam which turns a turbine to generate electricity.

- The magnitude of this energy conversion is about 108 times which is greater than that for chemical reactions.
- It is the world’s largest sources of emission free energy.
- Enrico Fermi who invented nuclear energy.
Definition of Nuclear Energy
- The energy released during a nuclear reaction due to the nucleus of an atom is called nuclear energy.
- It is also known as atomic energy because it can be considered to be coming from the atoms.
Types of Nuclear Reaction
- It can be obtained by two types of nuclear reactions:-
1. Nuclear Fission
2. Nuclear Fusion
1. Nuclear Fission
- The word ‘fission’ means to ‘ split up ‘ into two or more parts.
- The process in which the heavy nucleus of a radioactive atom splits up into smaller nuclei when bombarded with low energy neutrons, is called nuclear fission.
- It produced tremendous amount of energy.
- Nuclear fission is carried out by bombarding the heavy nuclei with low energy neutrons which are additionally called slow moving neutrons.
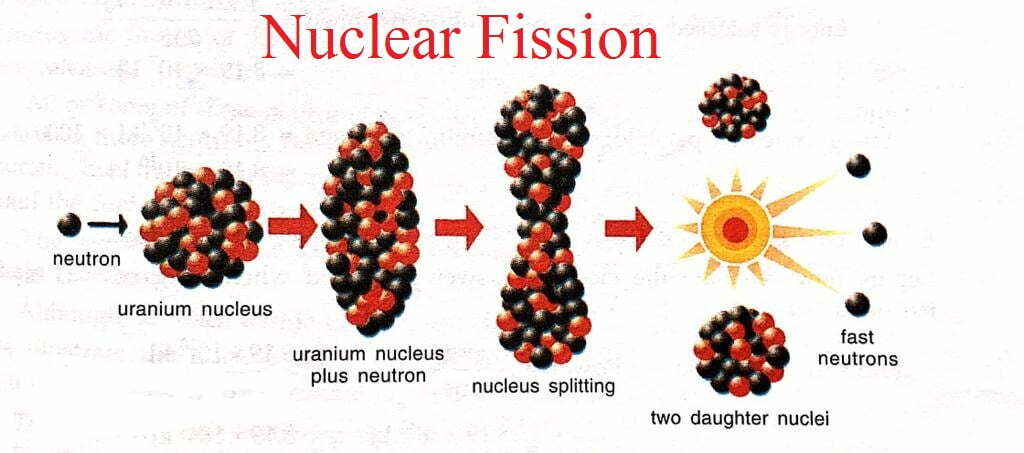
- For e.g. uranium-235 atoms are bombarded with slow moving neutrons, the heavy uranium nucleus breaks up to deliver two medium weight molecules, Barium-139 and Krypton 94, with the discharge of 3 neutrons. This fission reaction can be represented in the form of a nuclear equation as:-
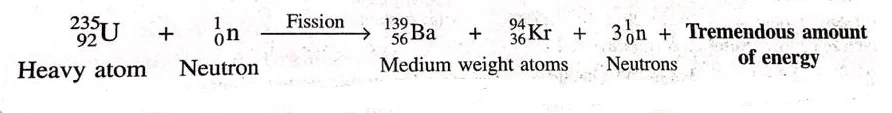
- In the fissioning of uranium, some mass of uranium disappears and a tremendous amount of energy is produced.
- The fission of 1 atom of uranium-235 produces 10 million times more energy than the energy produced by the burning of 1 atom of carbon from coal.
- This happens due to the conversion of mass into energy.
Control Nuclear Fission Reaction
- Reaction of nuclear fission is controlled by rods made of boron.
- Boron has a property that it can absorb neutrons.
- So, when a nuclear fission reaction is carried out in the presence of boron rods.
- The excess neutrons created during successive fissions of uranium- 235 atoms are absorbed by boron rods and hence not available to cause further fission.
- Because of this a controlled fission reaction of uranium- 235 takes place liberating heat energy at a slow, steady and manageable rate which can be used for generating electricity at a nuclear power plant.
Difference Between Controlled & Uncontrolled Nuclear Reaction
| S. NO. | Controlled Nuclear Reaction | Uncontrolled Nuclear Reaction |
| 1. | In a controlled fission reaction for every one neutron absorbed, only one neutron is available for further fission. | In an uncontrolled nuclear chain reaction, three neutrons are produced for each neutron absorbed. Each released neutron further produces three more neutrons and thus the chain reaction continues until explosion takes place. |
| 2. | The controlled fission reaction proceeds at a steady rate. The energy released can be utilized for useful purposes. | The uncontrolled fission reaction becomes explosive and the released energy cannot be used for useful purposes. |
Einstein’s Mass Energy Relation
- It states that mass and energy are equivalent, and are related by the equation:-
E = mc²
where,
E = Amount of energy
m = Mass Destroyed
c = Speed of light in vacuum
- Since the speed of light is very, very large, so an extremely large amount of energy is produced even if small mass gets destroyed.
- The destruction of mass happens in nuclear reactions with the liberation of tremendous amount of energy.
- The mass – energy equation, if we put the mass in kilograms ( kg ), and the speed of light in meters per second ( m/s ), then the energy will come in joules ( J ).
- For e.g. if a mass of 1 kg of any matter could be destroyed in a nuclear reaction, then the amount of energy produced would be given by Einstein’s equation as:-
E = mc²
E = 1 × ( 3 × 108 )²
E = 9 × 1016 J
Thus, 1 kg mass produces a huge amount of energy of 9 × 1016 J.
2. Nuclear Fusion
- The process of nuclear fusion was discovered in 1930s.
- The word ‘ fusion ‘ means ‘ to join ‘ & ‘ to combine ‘.
- The process in which two nuclei of lighter elements combine to form a heavy nucleus, is called nuclear fusion.
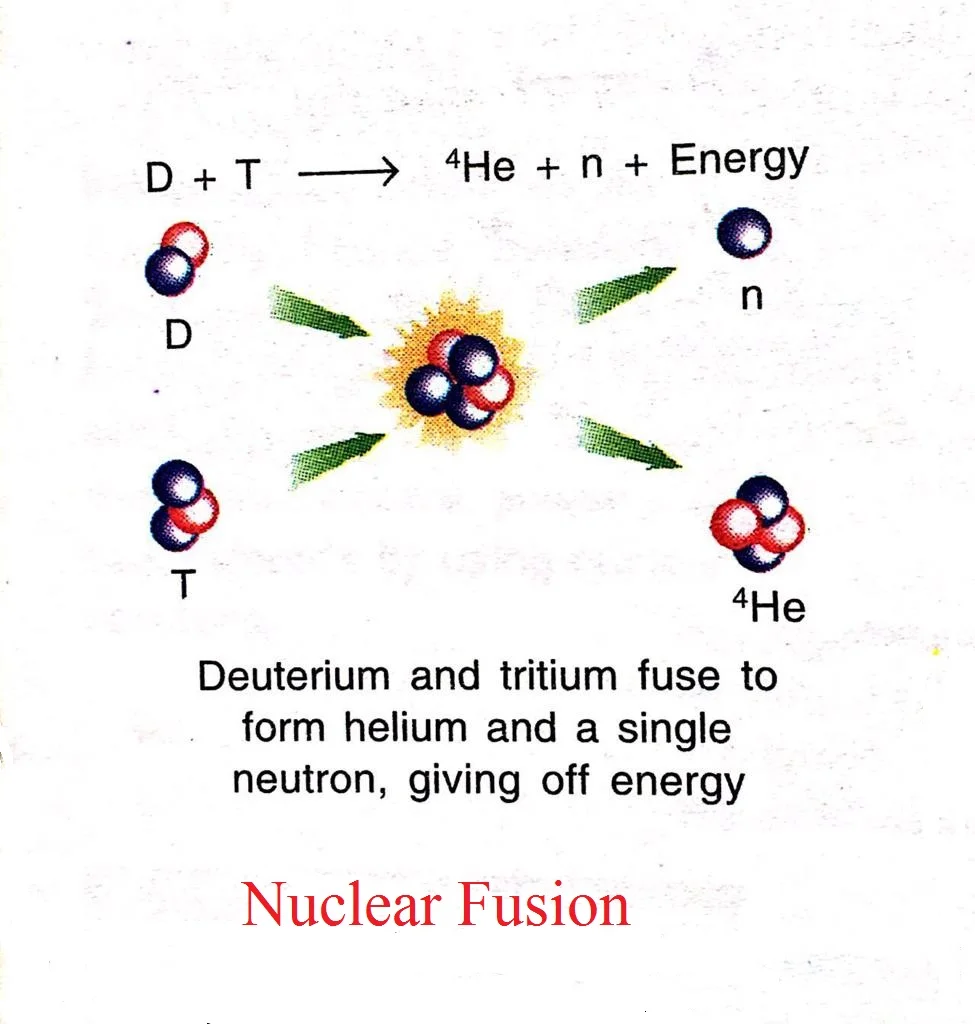
- The energy produced in a fusion reaction is much higher than that produced in a nuclear fission reaction.
- It takes place only at a very high temperature, about 4 – 15 million degrees. That is why nuclear fusion is also called thermonuclear reaction.
Typical Fusion reactions are:-
- Fusion reaction between two deuteron nuclei forming helium – 3 and a neutron.
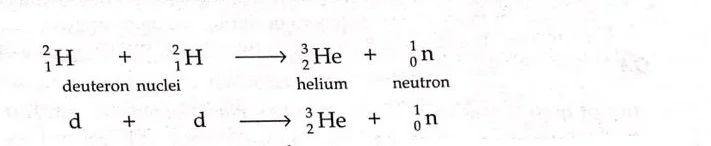
or
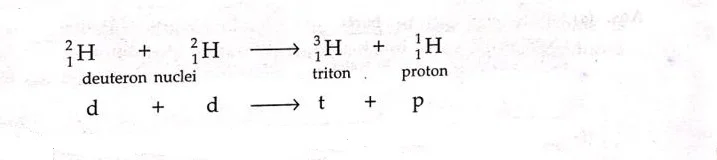
- Fusion reaction between two deuteron nuclei forming Triton and a proton.
or
where,
t= Triton, a nucleus containing one proton and two neutrons
d= deuteron, a nucleus containing one proton and one neutron
- Fusion reaction between two high speed colliding protons, leading to the formation of a neutron, a positron and a neutrino is also a nuclear fusion reaction. A large amount of energy is also released.
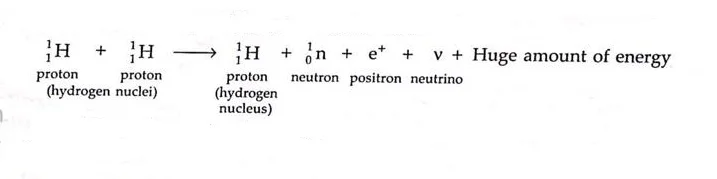
- In this reaction, one of the protons is converted into a neutron and a positron ( e+ ).
Differences Between Nuclear Fission & Nuclear fusion
| S.NO. | Nuclear Fission | Nuclear Fusion |
| 1. | In nuclear fission, a heavy nucleus breaks down into two or more lighter ones. | In nuclear fusion, two lighter nuclei combine to form a heavier nucleus. |
| 2. | Nuclear fission can take place at all temperatures. | Nuclear fusion takes place only at a very high temperature. |
| 3. | It needs a certain minimum amount of fuel. | There is no minimum limit to the amount of fuel. |
| 4. | Products of nuclear fission are generally radioactive. | Products of nuclear fusion are generally not radioactive. |
- Also read on touching the link:-
- Solar Cell in details
- Networking in details
- Green Concrete in details
- Geothermal Energy
- Heat Treatment
- Heat Transfer
- Energy
- Heat
- Light
About related to machine click on the link
Nuclear Energy Facts
- It produced 809 billion kilowatt hours of electricity in 2019.
- It provides 55% of America’s clean energy.
- This is most reliable energy source in America.
- It helps to power 29 states of U.S.
- This fuel is extremely dense.
Nuclear Energy Pros And Cons
| S. NO. | Pros | Cons |
| 1. | It produces immense power from tiny amounts of fuel. | Nuclear waste gives off dangerous radiation power much over 1,000 years. |
| 2. | Nuclear fuels will last for longer than fossil fuels such as coal, oil, and natural gas burned in other power plants. | Safety staring the waste is a huge problem. |
| 3. | Nuclear power plants produce clean energy that adds little to air and water pollution. | All of the nuclear waste made in the United states over the last 40 years could cover a football field, 15 feet deep. |
| 4. | Burning of fossil-fuel burning plants produce much pollution including acid rain. | Nuclear energy has several huge drawbacks. High level waste that produces strong radioactivity remains dangerous for hundreds-even thousands of years. |
| 5. | Nuclear energy workers wear special suits and respirators that become contaminated with low levels of radiation. | No nuclear waste storage area is totally safe for workers. Also the waste sites must be safely guarded to prevent intruders. |
| 6. | For every kilogram of nuclear energy produced, it is the same as using 1,000 pounds of coal. | Finally, as current storage areas fill up, we should manufacture new, safe sites for future nuclear wastes. |
Nuclear Energy Hazards
- The damaging and harmful effects associated with nuclear reactions are called nuclear hazards.
- Most substances engaged with the generation of nuclear energy are radioactive.
- These substances emit harmful radiation. These are called nuclear radiations.
- Nuclear radiations have very harmful effects on human bodies and vegetation.
- The harmful effects of the nuclear radiation on human body are of two types:-
1. Somatic effects
2. Genetic effects
1. Somatic effects
- The somatic effects involve damage to the body cells leading to the diseases such as cancer.
2. Genetic effects
- The genetic effects involve damage to the genes of the affected person.
- These effects are very serious and may be passed on to the next generation.
Safety Measures of Nuclear Energy
- The nuclear reactor should be located at a place far away from high population areas.
- The nuclear reactor should be provided with a thick coating of radiation absorbing material and enclosed in a thick concrete shield to prevent any radiation leakage.
- The building and the entire structure for the nuclear reactor should be earthquake-proof.
- We should start using non- conventional sources of energy.
Uses of Nuclear Energy
- It is used in the field of electric power generation.
- They are mostly used in the field of medicine.
- It is also used in the field of food & agriculture.
- It is used in industrial applications as well as consumer products.
- They are mostly used in the field of scientific research and space.
Advantages of Nuclear Energy
- It delivers a large amount of useful energy from a very small amount of a nuclear fuel.
- When the atomic fuel is stacked into the reactor, the atomic force plant can continue delivering power for a few years at a stretch. There is no requirement for placing in atomic fuel over and over.
- It doesn’t produce gases like carbon dioxide which contributes to greenhouse effect or sulphur dioxide which causes acid rain.
Disadvantages of Nuclear Energy
- The waste products of nuclear reactions are radioactive which keep on emitting harmful nuclear radiations for thousands of years. So, it is very difficult to store or dispose off nuclear wastes safely.
- Improper nuclear waste storage or disposal can contaminate the earth.
- There is the risk of accidents in nuclear reactors. Such accidents lead to the leakage of radioactive materials which can cause serious damage to the plants, animals and the nature.
- The high cost of installation of nuclear power plants and the limited availability of uranium fuel make the enormous scope utilization of nuclear energy prohibitive.
Let’s Know More
1. What is a nuclear reaction ?
Ans:- The reaction in which composition of the reacting nuclei changes to form nuclei of the lighter elements with a simultaneous release of a large amount of energy is called a nuclear reaction.
2. Differences between chemical and nuclear reaction.
Ans:-
| S.NO. | Chemical Reaction | Nuclear Reaction |
| 1. | In chemical reactions, new compounds are formed but the basic elements remain the same. | In nuclear reaction, new elements are formed due to the splitting or fusion of the nuclei. |
| 2. | In chemical reaction, only the electronic configurations change. | In nuclear reactions, composition of the nuclei changes. |
| 3. | The energy is released of the order of 100-200 kJ. | It releases very large amount of energy i.e approx. 1 million joules. |
| 4. | Chemical reaction can be reversed. | Nuclear reactions are irreversible. |
| 5. | The rate of a chemical reaction is affected by change in temperature and pressure. | There are no effect on the rate of a nuclear reaction when pressure / temperature are changed. |
3. What are the components of a nuclear energy power plant?
Ans:- A nuclear energy power plant consists of the following components:-
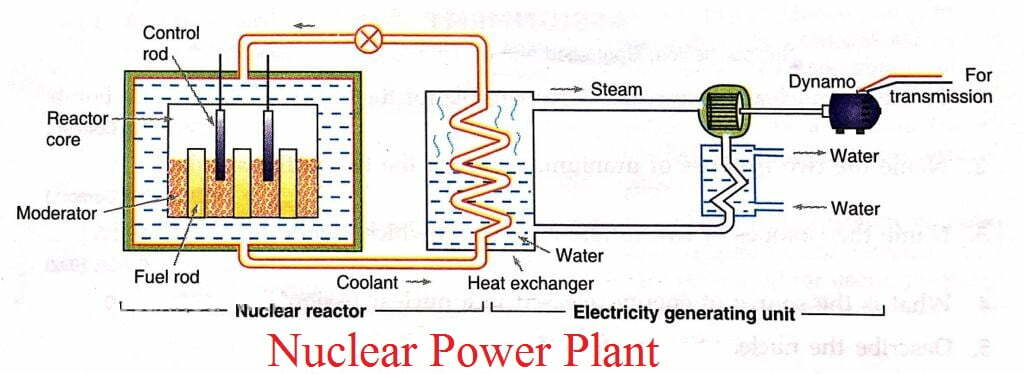
A. Nuclear reactor – Here, a controlled nuclear fission of a fissionable fuel such 235 92U is carried out.
B. Heat exchanger – The reactor is connected to a heat exchanger. Here, the heat produced in the reactor is transferred to water by circulating a coolant is pumped back to the reactor.
C. Steam turbine – The steam generated in the heat exchanger is used to run the steam turbine. The spent steam is sent back as hot water to the heat exchanger.
D. Electric generator ( Dynamo ) – The shaft of the steam turbine is connected to an electric generator. Electricity so produced is sent for transmission.
4. Why can’t nuclear fusion be carried out in a nuclear reactor ?
Ans:- Nuclear fusion reaction takes place at extremely high temperature. Such a higher temperature can’t be obtained for a reasonable length of time in a laboratory. Moreover, technically, there is no suitable material available which would be able to withstand such a high temperature. As a result, it is not possible to have a nuclear reactor based on the principle of nuclear fusion.
5. What are nuclear radiations?
Ans:- The rays and the particles emitted due to natural radioactivity and during nuclear fission are collectively called nuclear radiations.
- Most common nuclear radiations are
A. Alpha ( α ) particles
B. Beta ( β ) particles
C. Gamma ( γ ) particles
D. X – rays
E. High energy neutrons
6. How is nuclear energy waste generated ?
Ans:- Nuclear energy waste is generated in the following manner:-
A. From nuclear reactors
B. During mining of the radioactive materials
C. At nuclear fuel processing complexes
D. At laboratories and hospitals which use radioactive isotopes for research or medical purposes.
7. What is Hydrogen Bomb?
Ans:- Thermonuclear reactions are used for producing a weapon of mass destruction called Hydrogen Bomb.

