Table of Contents
Heat
Heat is a form of energy that is transferred from one body or system to another due to a temperature difference.
It’s a measure of the thermal energy that flows from an area of higher temperature to an area of lower temperature.
- The term Heat is a common noun.
- In English it is heat or warmth.
- In French chaleur, German warme, Latin calor etc.
- Heat is a form of energy that can be felt but cannot be seen.
- It causes the feeling of hotness or coldness.

- It always moves from a warmer place to a cooler place.
- These pass from a body at higher temperature to a body at lower temperature.
- Heat presents only at the boundary while the change takes place inside the system.
- It affects the movement of the molecules or particles in matter.
- The first scientist James Prescott Joule who discover that heat is a type of energy.
Definition of Heat
- Heat is a form of energy that flows from a hot body to a cold body.
- It is represented by the symbol Q.
- Its SI unit is joule. And another common unit is the calorie ( cal ).
- 1 cal = 4.184 J
Sign Convention
- If warmth flows from the system to surroundings, the quantity is said to be positive and if it flows from surroundings to system it is said to be negative.
In the other words,
- Heat received by system = +Q
- Heat rejected by system = −Q
Sources of Heat
- The following are the main sources of warmth:-
1. Sun
2. Fuels
3. Friction
4. Explosions
5. Electric Heater
6. Volcanic eruption
1. Sun

- The sun is the ultimate source of heat for all the living beings on the earth.
- Plants use the energy of the sun to prepare their food.
- Human beings are able to survive by the warmth of the sun.
2. Fuels

- It produce energy when they burn.
- In the fuels wood, coal, kerosene, petrol, and cocking gas are some of the commonly used.
- The warmth is produced by burning fuels is also used to derived the engines as well as to run the cars and to generate electricity.
3. Friction

- It is a kind of force that produces warmth.
- When we rub our palms, they become warm.
- A matchstick catches fires, when struck, due to friction.
4. Explosions

- This is phenomenon resulting from sudden release of energy which is dissipated by a blast wave, by translocation of the objects in the space or by warmth generation.
- It may be supersonic.
- It is not as fast as the speed of sound.
- They classify into three types:-
a. Atomic
b. Chemical
c. Mechanical
5. Electric Heater

- In cold weather, the electric heater provides warmth.
- We use geysers or water heaters to warmth the water.
- A filament is present in all these appliances.
- Electricity heats up the filament which emits warmth.
6. Volcanic eruption

- It produces internal heats from natural radioactivity.
- It happens when hot materials are thrown out of a volcano.
- Also read on touching the link:-
About related to machine click on the link
Characteristics of Heat
- It has no shape, no mass, no colour, no odour, no volume and no weight.
- It is an invisible from of energy.
- The presence of warmth is left only through its effect on matter.
- It might be transferred from one body to another.
- They flow in all directions.
- It might be transformed into other forms of energy. For example, they can be changed to light energy, on heating iron, it becomes red hot and emits light.
- They can also be converted into mechanical energy. For example, steam can move an engine.
- Conversely, other forms of energy can be transformed into warmth energy.
- For example, on burning a candle, the chemical energy is transformed into warmth and light energy.
- When we switch on an electric bulb, the electrical energy gets transformed into warmth and light energy.
Flow of Heat
Heat
HOT object → COLD object
Flow
- Suppose we keep two bodies in contact with each other.
- The flow of warmth from one body to another takes places.
- It is from hot object to a cold object.
- For example, mixing sugar in hot milk makes the spoon warm.
- When we touch the object, warmth flows from one body to the other.
- We feel cool when we touch the ice cubes. It is because our hand is hotter than the ice.
- On touching the ice cubes, warmth flows from our hand to the ice and we feel cold.
- Suppose we touch the water taken from a geyser, we feel hot.
- It is because the water taken from the geyser is hotter as compared to our normal body temperature.
- On touching this hot water, warmth flows from the water to our hand. As a result, we feel hot.
- When a hot body comes in contact with a cold body, it is observed after some time the hot body loses some of its warmth and the cold body also becomes less cold.
- This is because of the transfer of warmth in both objects.
Transfer of Heat
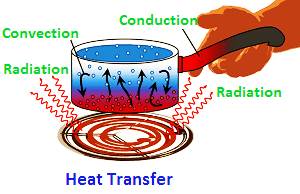
- The flow of warmth from one body to another or from one part of the body to another is called transfer of warmth.
- It can take place in three different ways:-
- For more in Details touch my link given below
- https://mechanicalnotes.com/heat-transfer-definition-modes-conduction-convection-radiation/
1. Conduction
2. Convection
3. Radiation
1. Conduction
- It is a process of transferring warmth from the hot end to the cold end from particle-to-particle of the medium.
- Thus, a medium is needed for the transfer of warmth by conduction.
- It mainly occurs in solids.
- The conductions that must be fulfilled so that the flow/transfer of warmth takes place by the conduction process:-
- A difference of warmth exists between two bodies or two ends of the same body.
- The two bodies at different points are in direct contact with each other.
2. Convection
- It is the process of transfer of warmth by the actual movement of the particles of the medium.
- Liquids and gases are mainly heated by convection as they are insulators of heat.
- Solids cannot be heated by convection as their particles are very tightly packed.
- Hence, they are unable to move.
- For examples, when you light a candle, the air above it gets heated and moves upwards.
3. Radiation
- It is a process of warmth transfer in which warmth passes directly from one body to other without affecting the medium.
- This means that no medium is needed for warmth transfer in the process of radiation.
- In vacuum, warmth transfer occurs only by the process of radiation.
- For example, we feel warm when we are in the sun.
Heat and Temperature
- Warmth and Temperature are two different concepts.
- Warmth causes temperature. Thus, there exists a cause effect relation between them.
- It is the cause and temperature is the effect.
- For example, when the body is heated, its temperature increases.
Differences Between Heat and Temperature
| S. NO. | Heat | Temperature |
| 1. | This is a form of energy. | It is the quantity that determines the degree of hotness or coldness of a body. |
| 2. | It is responsible for temperature. There can be no temperature without warmth. | Temperature is one of the effects of heat. |
| 3. | It raises the temperature of the body. It does not determine the direction of flow warmth. | It determines the direction of flow of warmth, i.e., from a body at a higher temperature to a body at a lower temperature. |
| 4. | The direction of transfer of warmth does not depend on the quantity of warmth. | The direction of transfer of warmth depends on the temperature. |
| 5. | It is measured in joule or calorie. | It is measured in degree Celsius( °C ) or degree Fahrenheit ( °F ). |
Application of Heat
Heat is used to prepare food and improve its flavor through processes like boiling, frying, baking, and smoking.
It is used to generate high-pressure steam to turn turbines and produce electricity or mechanical energy for vehicles.
- It can be used in heat therapy to relieve pain, reduce muscle stiffness, and promote healing.
- It is used in manufacturing for making products, such as glass, paper, and textiles, and for recycling plastic.
- It is applied to remove moisture from materials, whether it’s drying wet clothes, food products, or other manufactured goods.
- It is used to generate high-pressure steam to turn turbines and produce electricity or mechanical energy for vehicles.
- Incinerators use heat to burn and dispose of waste materials.
- It is used in various scientific experiments to study materials and processes.
- It can help to reduce joint stiffness and decrease tissue swelling.
- It is used in a bell in the circuit rings to warm of a fire.
- For cold application it is most often used for sport injury.
Disadvantage of Heat
It damages materials such as electronics, plastics, and wood when it’s at high temperature.
- High heat can lead to water evaporation, exacerbating water scarcity issues.
- It is difficult to control and can transfer easily.
- It can cause pavement melting, bridge damage, and other infrastructure issues.
- Extreme heat can cause discomfort, reducing productivity and affecting daily activities.
- Extreme heat can contribute to climate change, affecting ecosystems and biodiversity.
- Extreme heat can damage crops, reducing yields and affecting food security.
- High temperature can cause electronic equipment to fail or malfunction.
- Colling systems and air conditioning units consume a lot of energy to mitigate heat.
- Excessive heat can cause heat exhaustion, heat stroke, and other health issues.
Let’s known more
1. Define Thermal Equilibrium.
Ans:- When two bodies are in contact with each other and no warmth flows through them, they are said to be in the state of thermal equilibrium.
- The exchange of warmth between them is zero.
2. What is insulators of warmth?
Ans:- Those substance which do not allow warmth to pass through them easily. They are called insulators of warmth.
- For examples, wood, plastic, mud, cork, cotton, wool, etc.
3. What is conductors of warmth?
Ans:- Those substance which allow warmth to pass through them easily. They are called conductors of warmth.
- For examples, copper, silver, iron, mercury, etc.
4. Define Black surfaces.
Ans:- It absorb more radiation than white and shiny surfaces.


