Table of Contents
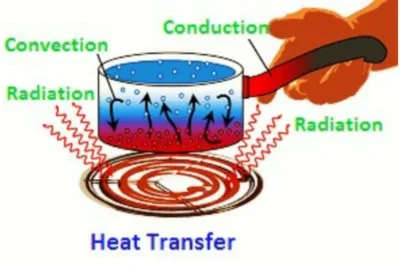
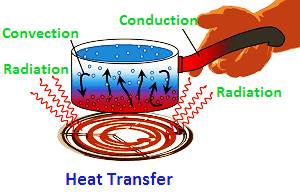
Heat Transfer
- The term heat is taken as synonymous to warm vitality.
- This utilization has its birthplace in the recorded understanding of warmth as a liquid ( caloric ) that can be moved by different causes, and that is additionally regular in the language of laymen and regular daily existence.
- Heat transfer consistently moves from a region of high temperature to another region of lower temperature.
- It changes the inside vitality of the two frameworks required by the principal law of thermodynamics.
- The trading of dynamic vitality of particles through the limit between two frameworks which are at various temperatures from one another or from their environment.
- The second law of thermodynamics characterizes the idea of thermodynamic entropy, by quantifiable warmth move.
- Thermal equilibrium is reached when all involves bodies and the surroundings reach the same temperature.
Definition of Heat Transfer
- It is defined as the transmission of energy from one region to another region to temperature difference.
or
- The flow of heat from one body to another or from one part of the body to another is called Transfer of Heat.
Also Read by touching the link:
Heat Treatment: Definition in Details.
Methods of Heat Transfer
- There are three methods of heat transfer namely :-
1. Conduction
2. Convection
3. Radiation
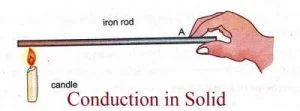
1. Conduction
- Conduction is the flow of heat in a substance due to the exchange of energy between molecules having more energy and less energy.
- In Conduction, energy exchange takes place by the kinematics motion or direct impact of molecules.
- Pure conduction is found only in solids.
Mechanism
- Lattic vibration ( 70 % ).
- Due to the free electron ( 30 % ).
Fourier’s Law of Heat Conduction
- It states that the rate of heat conduction through plain layer to the solid body is proportion to the temperature difference across the layer & heat transfer area inversely proportional to the thickness of layer.
Q = – KA × ( dt / dx )
K = Thermal conductivity
A = Area Square meter
( dt / dx ) = Temperature Gradient
Thermal conductivity
- Thermal conductivity is the ability of the material to conduct heat.
- Its unit is W / m K or °C.
Representative values of some thermal conductivity material
- For Solid
| Metal | K ( W / m K ) | State |
| Siliver | 429 | 20°C |
| Copper | 401 | 20°C |
| Pure Cu | 380 | 20°C |
| Brass | 110 | 20°C |
| Steel | 54 | 20°C |
| Stainless Steel | 16 | 20°C |
| Non-metal | ||
| Asbestos | 0.23 | 20°C |
| Plastic | 0.58 | 20°C |
| Wood | 0.17 | 20°C |
- For Liquid
| Liquid | K ( W / m K ) | State |
| Water | 0.60 | 20°C |
| Light Oil | 0.14 | 20°C |
- For Gases
| Gases | K ( W / m K ) | State |
| Dry air | 0.026 | 20°C |
| Steam | 0.025 | 20°C |
2. Convection
- The transfer of energy from one region to another due to the macroscopic bulk motion in a fluid, aeeded onto the energy transfer by conduction is called heat transfer by convection.
- It is possible only in the pressure of fluid medium.
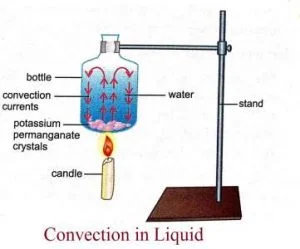
- Liquids and gases are mainly heated by convection as they are insulators of heat.
- Solids cannot be heated by convection as their particles are very tightly packed. Hence, they are unable to move.
- For examples, when you light a candle, the air above it gets heated and moves upwards.
- Similarly, we can see smoke from burning objects like incense stick, paper, etc move up. The particles are heated and moves upwards.
Characteristics of Convection
- For heating using the convection process, a material medium is necessary.
- It is not necessary that the particles of the medium should be compact.
- That is why liquids and gases can transmit heat through convection, but not solid.
- For transmission of heat through convection, actual movement of particles of that medium is essential.
Applications of Convection
- It causes land and sea breeze in coastal areas.
- In coastal areas, breeze blows from the sea towards the land during the day. It is called sea breeze.
- At night, breeze blows from the land towards the sea. It is called land breeze.
- The reason for this is as follows:-
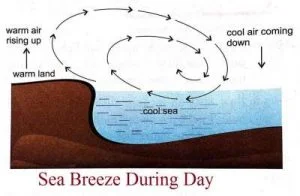
- During the day, the land surface heats up faster than the sea.
- Air above the land becomes warm and rises up.
- The cool air from the sea blows towards the land to take its place. This is called sea breeze.
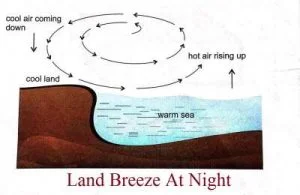
- At night, the land cools much faster than the sea.
- Thus, the sea is warmer than the land.
- The air above the sea is warm and rises up.
- A current of air blows from the colder land to warmer sea to take its place. This is called land breeze.
Newton’s Law of Cooling or Convection Law
- It states that the rate of heat flux transfer from surface to fluid is directly proportional to the difference in temperature.
q = hA ( Ts – T∞ )
Ts = Temperature of fluid
T∞ = Temperature of the surface
3. Radiation
- The heat transfer from one body to another without any transmitting medium is known as Radiation.
- It is an electromagnetic wave phenomenon.
- For examples, we feel warm when we are in the sun, we feel warm when we sit near a fire.
- Black and dull surfaces absorb more heat radiations as compared to white and shiny surfaces.
Properties of Radiation / Characteristics
- It doesn’t require any medium.
- Rate of emission increase with temperature level.
- It travel with the speed of light.
Applications of Radiation
- The bottom part of some cooking utensils is painted black so that it can absorb more heat and the food gets cooked rapidly.
- Room heaters are provided shiny surface reflects and absorbs very little heat.
Fundamental Law of Heat Transfer
- There are two law of heat transfer :
1. Fundamental law
i. Law of conversation of mass
ii. Newton Law of motion
iii. Law of Thermodynamics
2. Subsidiary law
i. Fourier Law of Heat Conduction
ii. Newton’s Law of Cooling
iv. Law of Radiation