Table of Contents
Introduction
Cement is a binder material that is used to mix with various construction materials like sand, gravel, and water to form concrete and mortar.
It is a fine gray powder that is made from the burning of clay and lime.
- It consists of calcium, silicon, aluminum, and iron compounds, which are essential for its binding properties.
When cement is mixed with water, it creates a paste, and this paste binds together other materials like sand and crushed rocks to form mortar and concrete.
- It is used in buildings for sticking bricks or stones together or for making very hard surfaces.
- It was first invented by Egyptians.
- The manufacturing of cement was started in England around 1825.
- Joseph Aspdin manufactured it and calls it Portland cement.

- Portland cement because when it hardens, it produces a material resembling stone from the quarries near Portland in England.
- It obtains by burning together a mixture of naturally occurring argillaceous and calcareous materials at high temperature.
- The product obtained on burning called clinker.
- Clinker cools and grinds to the required fineness to produce a material known as cement.
Definition of Cement
Cement is a binder, a chemical substance used in construction for creating concrete and mortar.
- It is an extremely fine material having adhesive and cohesive properties which provide a binding medium for the discrete ingredients.
Chemical Composition of Cement
- The materials use for the manufacturing consist mainly of Lime, Silica, Alumina and Iron oxide.
- Oxides presents in the raw materials when subjects to high clinkering temperature combined with each other to form complex compounds.
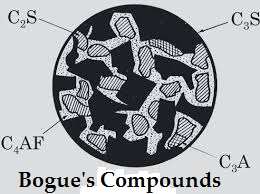
- The identification of the major complex compounds is based on R.H. Bogue’s work and hence these are calls Bogue’s compounds.
- In addition to the four major components, there are mainly minor compounds form in the kiln.
- The influence of these minor compounds on the properties of cement or hydrated compounds is not sufficient.
- Two of the minor oxides namely K2O and Na2O referrers to as alkalies in cement are of some importance.
| Constituents | Percentage | Average Percentage |
| Lime ( CaO ) | 62 – 67 % | 62 |
| Silica ( SiO2 ) | 17 – 25 % | 22 |
| Alumina ( Al2O3 ) | 3 – 8 % | 5 |
| Calcium Sulphate ( CaSO4 ) | 3 – 4 % | 4 |
| Iron Oxide ( Fe2O3 ) | 3 – 4 % | 3 |
| Magnesia ( MgO ) | 0.1 – 3 % | 2 |
| Sulphur | 1 – 3 % | 1 |
| Soda and Potash ( Na2O + K2O ) | 0.5 – 1.3 % | 1 |
Bogue’s compounds:-
| Name | Chemical Formula | Percentage |
| Tricalcium Silicate ( C3S ) | 3CaOSIO2 | 30 – 50 % |
| Dicalcium Silicate ( C2S ) | 2CaOSiO2 | 20 – 45 % |
| Tricalcium Aluminate ( C3A ) | 3CaOAl2O3 | 8 – 12 % |
| Tetracalcium Alumino Ferrite ( C4AF ) | 4CaOAl2O3Fe2O3 | 6 – 10 % |
Representation of the composition of Bogue’s compounds is as follows :-
Functions of Various Cement Ingredients
1. Lime ( CaO )
- Important Ingredient.
- Proportion should carefully maintain.
- Excess lime makes it unsound and causes it to expand & disintegrate.
- Lime deficiency decreases strength and cause quick setting.
2. Silica ( SiO2 )
- Important Ingredient.
- Provides strength.
- Excess quantity increases strength but also increases setting time.
3. Alumina ( Al2O3 )
- Imparts quick setting.
- Lowers the clinkering temperature.
- Use of excess amount decreases strength.
4. Calcium Sulphate ( CaSO4 )
- This ingredient is in the form of gypsum.
- Increases the initial setting time.
5. Iron Oxide ( Fe2O3 )
- Gives colour.
- Provides hardness.
- Imparts strength.
6. Magnesia ( MgO )
- Small amount provides hardness and colour.
- High content makes unsound.
7. Sulphur ( S )
- Small amount is useful in making sound cement.
- Excess content causes unsoundness.
8. Alkalies
- Excess content causes alkali -aggregate reaction and efflorescence.
Basic Properties of cement Compounds
- C3S and C2S which together constitute about 70 – 80 %.
- C3S produces at a faster rate of reaction accompanied by greater heat evolution and imparts early strength.
- A higher percentage of C3S results in rapid hardening with an early gain in strength with a higher heat of hydration.
- C2S hydrates and hardens slowly and provides much of the ultimate strength.
- A higher percentage of C2S results in slow hardening, less heat of hydration and greater resistance to chemical attack.
- C3A is characteristically fast reacting with water and may lead to an immediate stiffening of paste and this process is known as Flash set.
- The role of gypsum added in the manufacture of cement is to prevent such a fast reaction.
- C3A provides weak resistance against Sulphate attack and it’s contribution to the development of strength is perhaps less significant than that of C3S and C2S.
- Like C3A, C4AF also hydrates rapidly but it individual contribution to the overall strength is insignificant. However, it is more stable than C3A.
Hydration of Cement
- The chemical reactions that take place between cement and water is known as hydration of cement.
- This reaction is exothermic which liberates a considerable quantity of heat and this liberates heat is called as heat of hydration.
- The hydration process is not an instantaneous one.
- The reaction is faster in the early periods and continues indefinitely at a decreasing rate.
- During the hydration, C3S and C2S react with water and calcium silicate hydrate forms along with calcium hydroxide [ Ca ( OH )2 ].
- Calcium silicate is the most important product of hydration and it determines the good properties of concrete.
2C3S + 6H → C3S2H3 + 3Ca ( OH )2
- and
2C2S + 4H → C3S2H3 + Ca (OH )2
- It can see from the above reactions that C3S produces a less quantity of calcium silicate and more quantity of calcium hydroxide than that forms in the hydration of C2S.
Types of Cement
- There are different types of cement as classified as follows :-
a. 33 Grade
b. 43 Grade
c. 53 Grade
2. Rapid Hardening Cement
3. Extra Rapid Hardening Cement
4. Low Heat Portland Cement
5. Portland Slag Cements
6. Portland Pozzolana cement
7. Sulphate Resisting Portland Cement
8. White Portland Cement
9. Coloured Portland Cement
10. Hydrophobic Cement
11. High Alumina Cement
12. Super Sulphated Cement
13. Special Cement
a. Masonry Cement
b. Air Entraining Cement
c. Expansive Cement
d. Oil Well Cement
1. Ordinary Portland Cement ( OPC )
- Also known as setting cement.
- It is the basic Portland cements and manufactures in large quantities than all other cements.
- It is presently available in three different grades viz. C33, C43 and C53.
- The numbers 33, 43 and 53 correspond to the 28 days compressive strength of cement as obtain from standard tests on cement -sand mortar specimens.
- It uses in general concrete construction.
2. Rapid Hardening Cement ( RHC )
- It is finer than ordinary Portland cements.
- It contains more C3S and less C2S than OPC.
- The one day strength of this is equal to the 3 days strength of OPC with the same water cement ratio.
- The main advantage of rapid hardening cement is that shuttering may be removed much earlier, thus saving considerable time and expenses.
- RHC is also used for road work where it is imperative to open the road traffic with the minimum delay.
3. Extra Rapid Hardening Cement ( ERHC )
- It is obtains by mixing calcium chloride with RHC.
- Addition of CaCl2 imparts quick setting properties in extra RHC.
- The acceleration of setting, hardening and evolution of heat in the early period of hydration makes this cement very suitable for concreting in cold weathers.
- The 1 or 2 day strength of extra RHC is 25% more than that of RHC.
- The gain of strength disappears with age and 90 days strength of extra RHC and RHC are nearly the same.
- Use of extra RHC prohibits in prestressed concrete construction.
Also read : Green Concrete
4. Low Heat Portland Cement
- It is a Portland cement which obtains by reducing the more rapidly hydrating compounds, C3S and C3A and increasing C2S.
- The heat of hydration of low -heat cement shall be as follows :-
- 7 days – not more than 65 calories per gm
- 28 days – not more than 75 calories per gm
- Since, the rate of gain of strength of this cements is slow, hence adequate percaution should be taken in this use such as with regard to removal of formwork, etc.
- LHC uses in massive construction works like abutments, retaining walls, dams, etc. where the rate at which the heat can be lost at the surface is lower than at which the heat is initially generated.
- It has low rate of gain of strength, but the ultimate is practically the same as that of OPC.
5. Portland Slag Cement ( PSC )
- PSC makes by intergrinding portland cements clinker and granulated blast furnace slag.
- The proportion of the slag being not less than 25 % or more than 65 % by weight of cement.
- The slag should granulate blast furnace slag of high lime content, which produces by rapid quenching of molten slag obtain during the manufacture of pig iron in a blast furnace.
- In general blast furnace slag cement finds to gain strength more slowly than the ordinary portland cement.
- The heat of hydration of portland blast furnace slag cements is lower than that of OPC. So, this cement can use for mass concreting but is unsuitable for cold weather.
- It has fairly high sulphate resistance, rendering it suitable for use in environments exposed to sulphates.
- It uses for all purpose for which ordinary portland cement uses.
- Because of its low heat evolution, it can use in mass concrete structure such as dams, foundations and bridge abutments.
6. Portland Pozzolana Cement ( PPC )
- It can produce either by grinding together portland cement clinker and pozzolana with the addition of gypsum or by blending uniformly portland cement and fine pozzolana.
- PPC produces less heat of hydration and offers great resistance to the attack of impurities in water than OPC.
- PPC is particularly useful in marine and hydration constructions, and other mass concrete structures.
- The disadvantage of using PPC is that the reduction in alkalinity reduces the resistance to corrosion of steel reinforcement.
- This cement has higher resistance to chemical agencies and to sea water because of absence of lime.
- It evolves less heat and its initial strength is less but final strength is equal to OPC.
- It has lower rate of development of strength than OPC.
7. Sulphate Resisting Cement ( SRC )
- The portland with low C3A and C4AF and ground finer than OPC is known as sulphate resisting cements.
- This cement is ” Sulphate resistant ” because the disintegration of concrete causes the reaction of C3A in harden cements with a sulphate salt from outside is inhibited.
- It uses in marine structures, sewage treatment works, and in foundations and basements where soil is infests with sulphates.
- However, recent research indicates that the use of sulphate resisting cement is not beneficial in environments where chlorides are present.
8. White Portland Cement
- The process of manufacturing white cement is the same but the amount of iron oxide which is responsible for grayish colour is limited to less than 1 %.
- Sodium Alumino Ferrite adds to act as flux in the absence of iron oxide.
- The properties of white cement is nearly same as OPC.
- Whitness of white cement measures ISI scale or Hunter’s scale.
- Grey colour of OPC is due to the presence of iron oxide. Hence in white cement, Fe2O3 is limited to 1 %.
9. Coloured Portland Cement
- It is a type of mixture of white cements along with the desired colour and color determines its original presence.
- The cost of this cement is high than OPC.
10. Hydrophobic Cement
- It obtains by intergrinding OPC with 0.1 – 0.4 % of water repellant film -forming substance such as oleic acid or stearic acid.
- The properties of hydrophobic cements are nearly the same as that of OPC.
- The cost of this cement is high than OPC.
11. High Alumina Cement ( HAC )
- It is very different in composition from portland cements.
- It characterizes by its dark color , high early strength , high heat of hydration and resistance to chemical attack.
- The raw material uses for its manufacture consists of limestone and bauxite which is a special clay with high alumina content.
- It has a good resistance against sulphates and some dilute acids, and particularly recommend in marine environments.
- It has an initial setting time of 4 hrs and final setting of about 5 hrs.
- High alumina cement is very expensive to manufacture.
- It uses where early removal of the framework is required.
- Its rapid hardening properties arise from the presence of calcium aluminate.
- This must not mix with any other types of cements.
12. Super Sulphated Cement ( SSC )
- It obtains from well granulated blast furnace slag, calcium sulphate and OPC.
- It is ground finer than OPC.
- This has low heat of hydration.
- It is used for construction of dams and other mass concreting works.
- It has high resistance to chemical attack.
13. Special Cement
- It has some specific function such as altering the setting or hardening behavior of a concrete.
Field Tests For Cements
- Colour : Grey color with a light greenish shade.
- Physical properties : Cement should feel smooth when rubs in between the fingers.
- If hand is inserted in a bag, it should feel cool.
- If a small quantity of cement throw in a bucket of water, it should sink and should not float on the surface.
- Presence of lumps : Cement should free from lumps.
Laboratory Test of Cements
1.Chemical composition
2. Normal ( standard ) consistency
3. Initial and final setting time
4. Soundness
5. Strength
6. Fineness
7. Heat of hydration
8. Specific gravity
1.Chemical Composition Test
- This is the type of test in which the ratio of percentage of lime to percentage of silica, alumina and iron oxide, when calculated by the formula ( CaO – 0.7SO3 ) / ( 2.8SiO2 + 1.2Al2O3 + 0.65Fe2O3 ) shall not be greater than 1.02 and not less than 0.66.
2. Normal ( standard ) Consistency Test
- The normal consistency of a cement paste is defined as that consistency which will permit a Vicat plunger having 10mm diameter and 50mm length to penetrate a depth of 33 to 35mm from the top of the mould.
3. Initial and Final setting time Test
- Initial setting time test is a test that is the time elapses between the moment that the water is added to the cement, to the time that the paste starts losing its plasticity.
- Final setting time test is a test that is the time elapses between the moment that the water is added to the cement and the time when the paste has completely losses its plasticity and has attains sufficient firmness to resist certain definite pressure.
4. Soundness Test
- Soundness of cement indicates that the cement paste, once it has set, does not undergo change in volume causing concrete to crack.
5. Strength Test
- Strength test is determined by compressive strength test and tensile strength test.
6. Fineness Test
- Fineness test is the major of the size of the cement particles in the term of specific surface.
7. Heat of hydration Test
- Heat evolves during hydration of cement, the amount being dependent on the relative quantity of clinker compounds.
- The apparatus uses to determine the heat of hydration of cement is known as Calorimeter.
8. Specific gravity Test
- It is obtained by Le Chatelier’s flask.
Specific gravity = Weight of Cement / Weight of Displaced volume of Liquid
Advantages of Cement
- There are nine advantages of cement, which are as follows:
1. It has high compressive strength to bear heavy loads.
2. It provides the most durability and longevity for long-lasting structures.
3. It is non-combustible and can help prevent the spread of fires.
4. It is a cost-effective building material compared to other materials like steel or wood.
5. It also provides a smooth surface for roads and a strong, durable bond to other materials.
6. It is resistant to pests and rodents, which helps to reduce the risk of damage and infestation.
7. It can be finished in a variety of ways, allowing for creative and attractive architectural designs.
8. It has excellent workability, making it easy to mix with other materials and to lay, mold, and finish during construction.
9. Cement is highly resistant to wear and tear and harsh environmental conditions, ensuring a long service life and reducing the need for frequent repairs.
For better understanding watch this video clip
https://www.youtube.com/watch?v=dyxL_BvkhJg



WoW nice sir 👍👍